Abstract
Background: Dysfunction of T cells, NK cells and other immune subsets is common in patients (pts) with CLL. Venetoclax (VEN), a BCL-2 inhibitor and obinutuzumab (OBIN), a CD20 monoclonal antibody (mAb) are approved for pts with CLL (Fischer, NEJM 2019). Atezolizumab, a PD-L1 checkpoint inhibitor (CPI), is approved for melanoma, lung cancer and other solid tumors. Preclinical studies showed synergy of VEN and CD20 mAb with CPI (Kohlhapp, Cancer Discovery 2021; Westin, Lancet Oncology 2014). Clinical studies showed activity of PD1 inhibition in pts with Richter's transformation, but not CLL (Ding, Blood 2017; Jain, ASH 2018). To our knowledge, no prior study has evaluated PD-L1 inhibition in pts with CLL, nor combined CPI, VEN and OBIN. We hypothesized that combined VEN, OBIN and atezolizumab will be synergistic.
Methods: This is an investigator-initiated Phase II trial of combined VEN, OBIN and atezolizumab in pts with previously untreated CLL meeting 2008 IWCLL treatment criteria (NCT02846623). Eligibility criteria included age ≥18 years, adequate organ function (total bilirubin ≤1.5 x ULN, ALT and AST ≤2.5 x ULN, creatinine ≤1.5 x ULN). OBIN was given at a flat dose of 100mg IV Cycle (C)1 Day (D)1, 900 mg C1D2, 1000mg on C1D8, 1000mg on C1D15 and then 1000mg on C2-9 D1. Atezolizumab was given at a flat dose of 1680 mg IV (split over 2 days) on C1D3-4 and then C2-9D1-2. VEN was initiated at the start of C3 with the weekly dose-escalation (20mg daily to a target dose of 400mg daily) and continued daily until end of C14 (total 12 cycles of VEN). All pts stopped therapy at the end of C14. Response assessments were done with CT imaging and bone marrow aspirate/biopsy with MRD assessment (multi-color flow cytometry; sensitivity 10 -4) at the end of C2 (prior to VEN initiation), end of C6, end of C9, and end of C14.
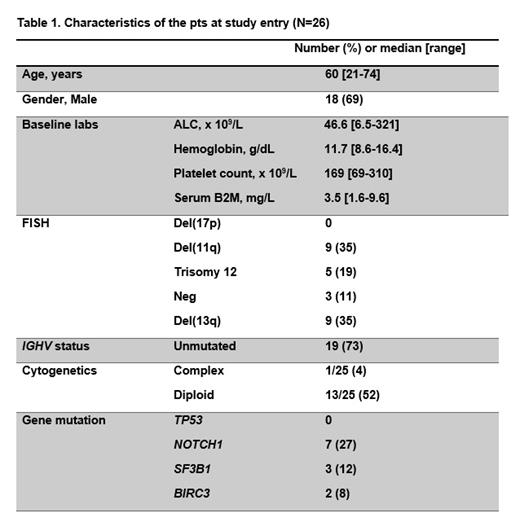
Results: From July 2019 to December 2020, a total of 26 pts were enrolled. The median age was 60 years (range, 21-74). The baseline characteristics are shown in Table 1. A total of 19/26 (73%) had unmutated IGHV gene. Though the study did not restrict pts with del(17p) or mutated TP53, no pt in the current cohort had del(17p)/ mutated TP53. A total of 14 (54%) pts had a baseline lymph node >5cm. The median follow-up is 13.3 months.
One pt came off study in C1 (details below). A total of 25 pts initiated VEN. The TLS risk categories at the start of C1 were high (n=9, 36%), medium (n=12, 48%), and low (n=4, 16%). After 2 cycles of OBIN and atezolizumab (prior to VEN initiation), the majority of pts had downgrading of TLS risk category [high (n=2, 8%), medium (n=3, 12%), and low (n=20, 80%)].
After C6 (about 3 cycles of VEN 400mg daily), bone marrow undetectable (U)-MRD rate was 19/25 (76%); 4/25 (16%) had low+ MRD and 2/25 (8%) had high+ MRD. After C9 (about 6 cycles of VEN 400mg daily), among the 21 pts (4 pts have not reached this time-point), the bone marrow U-MRD rate was 18/21 (86%); 2/21 (10%) had low+ MRD and 1/21 (5%) had high+ MRD. A total of 14 pts completed C14 (9 pts have not reached this time-point; 2 pts came off study prior to completing C14, details below); 13/14 (93%) achieved bone marrow U-MRD and 1/14 (7%) has low+ MRD. No patient had disease progression or MRD relapse so far. One pt died (details below).
Three pts came off study (one developed retroperitoneal hematoma after receiving enoxaparin for DVT in C1; one developed CPI-induced colitis and removed from the study in C10; one died from COVID-19 pneumonia in C14 while in bone marrow U-MRD remission).
Grade 3-4 neutropenia occurred in 14/26 (54%) pts. Grade 3 thrombocytopenia occurred in 5/26 (19%) pts; no pt had G4 thrombocytopenia. A total of 4 pts developed CPI-induced toxicities (colitis, G3, n=1; mucositis, G3, n=1; nephritis, G2, n=1; myositis, G2, n=1). A total of 10/25 (40%) pts had dose reduction of VEN, the majority due to neutropenia. Atezolizumab was discontinued early in 3 pts due to CPI-induced toxicities. Laboratory correlative studies including scRNAseq and CyTOF are ongoing.
Conclusions: Treatment with combined VEN, OBIN and atezolizumab leads to high rate of early U-MRD remission with 76% bone marrow U-MRD remission at the end of C6 (about 3 cycles of VEN 400mg daily). Four pts had CPI-induced toxicities. The enrollment in this trial continues and updated data and correlative studies will be presented at the ASH meeting.
Jain: Pfizer: Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Precision Biosciences: Honoraria, Research Funding; Aprea Therapeutics: Research Funding; AstraZeneca: Honoraria, Research Funding; Servier: Honoraria, Research Funding; Incyte: Research Funding; Pharmacyclics: Research Funding; Genentech: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; TG Therapeutics: Honoraria; Janssen: Honoraria; Beigene: Honoraria; Fate Therapeutics: Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding. Ferrajoli: Janssen: Other: Advisory Board ; AstraZeneca: Other: Advisory Board, Research Funding; BeiGene: Other: Advisory Board, Research Funding. Yilmaz: Daiichi-Sankyo: Research Funding; Pfizer: Research Funding. Thompson: AbbVie: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Gilead: Other: Institution: Advisory/Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Pharmacyclics: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Adaptive Biotechnologies: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding, Expert Testimony; Genentech: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Amgen: Other: Institution: Honoraria, Research Grant/Funding. Konopleva: Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; KisoJi: Research Funding; Stemline Therapeutics: Research Funding; Sanofi: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Cellectis: Other: grant support; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Calithera: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Agios: Other: grant support, Research Funding. Neelapu: Takeda Pharmaceuticals and related to cell therapy: Patents & Royalties; Kite, a Gilead Company, Bristol Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics (Cogent Biosciences), Allogene, Precision BioSciences, Acerta and Adicet Bio: Research Funding; Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene, Kuur, Incyte, Precision BioSciences, Legend, Adicet Bio, Calibr, and Unum Therapeutics: Other: personal fees; Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, Unum Therapeutics and Bluebird Bio: Honoraria. Takahashi: Symbio Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy; Novartis: Consultancy; GSK: Consultancy. Burger: TG Therapeutics: Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Beigene: Research Funding, Speakers Bureau; Novartis: Other: Travel/Accommodations/Expenses, Speakers Bureau; Pharmacyclics LLC: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Gilead: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; AstraZeneca: Consultancy; Janssen: Consultancy, Other: Travel/Accommodations/Expenses, Speakers Bureau. Khoury: Stemline Therapeutics: Research Funding; Kiromic: Research Funding; Angle: Research Funding. Kantarjian: Jazz: Research Funding; NOVA Research: Honoraria; Novartis: Honoraria, Research Funding; KAHR Medical Ltd: Honoraria; Precision Biosciences: Honoraria; Amgen: Honoraria, Research Funding; Astra Zeneca: Honoraria; AbbVie: Honoraria, Research Funding; Ipsen Pharmaceuticals: Honoraria; Pfizer: Honoraria, Research Funding; Astellas Health: Honoraria; Aptitude Health: Honoraria; Taiho Pharmaceutical Canada: Honoraria; Immunogen: Research Funding; Daiichi-Sankyo: Research Funding; BMS: Research Funding; Ascentage: Research Funding. Wierda: Karyopharm: Research Funding; Miragen: Research Funding; Acerta Pharma Inc.: Research Funding; Cyclacel: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Sunesis: Research Funding; Juno Therapeutics: Research Funding; Gilead Sciences: Research Funding; AstraZeneca: Research Funding; Genentech: Research Funding; Loxo Oncology, Inc.: Research Funding; Janssen: Research Funding; Xencor: Research Funding; GSK/Novartis: Research Funding; KITE Pharma: Research Funding; Genzyme Corporation: Consultancy; AbbVie: Research Funding.
Atezolizumab is not approved for CLL